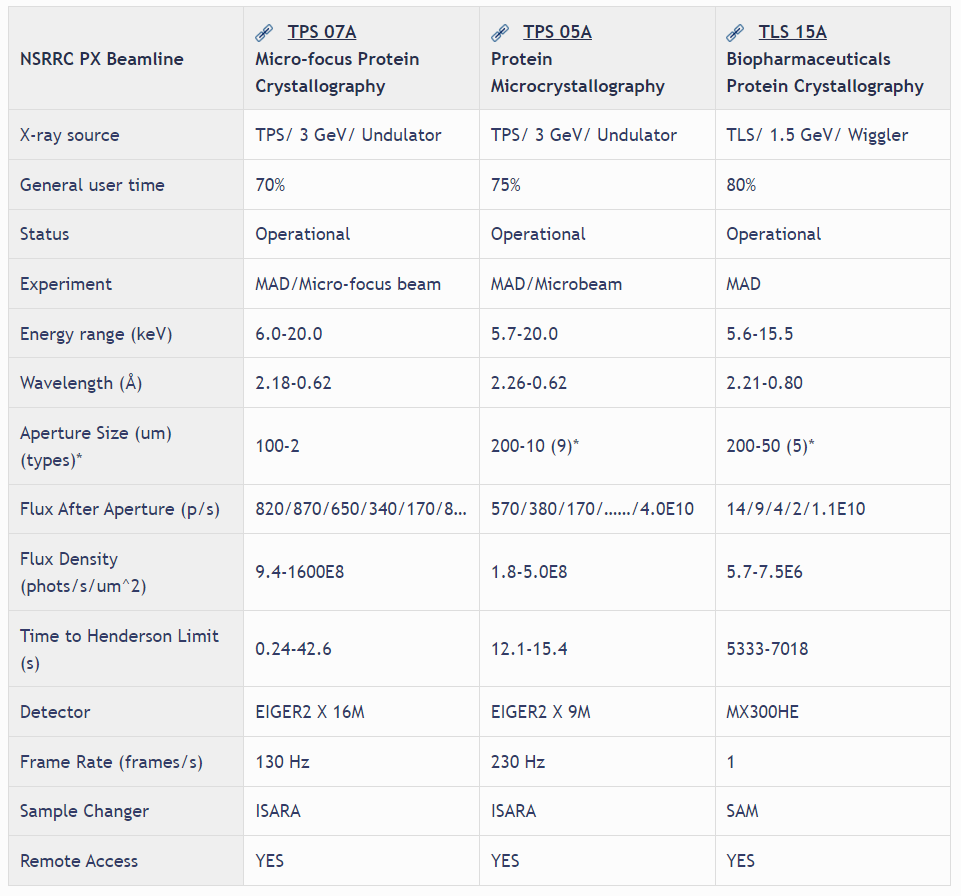
The routine use of synchrotron radiation for single crystal diffraction study in the past decades has revolutionized macromolecular structural biology. However, crystals of important macromolecules, such as membrane proteins and viruses, are usually small in sizes and have poor diffraction quality. Advances in synchrotron radiation sources, detectors, and software are necessary to tackle these challenging problems. TPS 05A aims for difficult structures as well as routine data collection. The X-ray source of TPS 05A is a three meters long in-vacuum undulator (IU22), producing a high-brilliant X-ray beam. The X-rays are monochromated by a liquid-nitrogen-cooling Si double-crystal monochromator, and focused by a pair of Kirkpatrick–Baez mirrors. The focused beam size at the sample is 65 μm (H) x 36 μm (V) with photon flux of 1.1 x1013 photons/s at 12.4keV. Apertures are used to collimate the beam in the range of 50–5 μm. The beam divergence at the sample is less than 500 μrad (H) and 100 μrad (V), and the energy range is from 5.7 to 20 keV (wavelength 2.175-0.62 Å). Equipped with a high speed CCD area detector and a micro-diffractometer, TPS 05A is capable of not only shutterless data collection for much higher throughput and helical scan to mitigate the radiation damage but also grid scan to find the best diffraction areas or to locate micro crystals. The optional mini-κ goniometer of the high precision micro-diffractometer enables crystal reorientation. A robotic sample changer for automatically sample mounting and centering in installed, making the data acquisition more efficient. Substantial user-support, remote access and mail-in service are also provided.
Beam properties of PX beamlines at the NSRRC